By Siha Hoque
The human body is prepared to face all manner of injury and illnesses through a series of general first-line responses, followed by the more specialised and specific if required.
Haemostasis is the body’s response to any injury where blood is being lost - to stop the bleeding (‘haemo’ comes from the Greek ‘heima’, meaning ‘blood’, ‘stasis’ meaning ‘stop’). Human blood consists of four general components; red blood cells, white blood cells, plasma and platelets. As you may know, platelets are the main agent in blood clotting, however there is a more complex process resulting in their activation - the four stages to haemostasis:
- Blood vessel constriction
- Formation of a platelet ‘plug’
- The coagulation cascade
- The formation of a fibrin plug
See above: A thrombus - or 'developed' blood clot.
Stage One: Vasoconstriction:
Vasoconstriction is where the diameter of the blood vessels reduces due to a contraction in the muscular tissue of the vessel walls, and the opposite for vasodilation. This can be triggered by a number of environmental stimuli, including a drop in temperature, certain medications and hormonal changes.
In this case, it is initiated by vascular spasms and the release of chemicals from the vessel walls’ endothelial cells, known as endothelin and thromboxane A2. This results in the exposure of a structure known as the extracellular matrix (ECM), which is a web of carbohydrates and proteins outside of cells, which they can adhere to as a physical scaffold and serves as a communication system too.

See above: Chemical structure of endothelin-1.

See above: Platelets in their natural state (left) and in pseudopodial shape (right).
Stage Two: Formation of the Platelet Plug:
The ECM releases chemicals which serve as inflammatory markers, leading to the adhesion of platelets and their accumulation at the site of the injury. This is a complex chain reaction, beginning with the platelets being driven towards the site of the injury by other cells, such as erythrocytes. A glycoprotein known as the von Willebrand Factor aids this process by combining platelets to the collagen at the site of the damage.
These accumulating platelets then undergo very specific changes, after the release of chemicals such as cytoplasmic granules and factors (coming up later), to reach a pseudopodial shape, shown below. This shape is better for plugging the site of the wound, spreading chemicals to other platelets and adhering to the site, due to their larger surface area and compactness. This is the primary platelet plug.
Stage Three: The Coagulation Cascade:
This is a series of enzyme-based reactions which occur as a second line of defence where the primary platelet plug is not enough to stabilise the bleeding at the site of a wound. There are three main approaches, or pathways, the body can use, and they are the extrinsic, intrinsic and common pathways.
Every pathway is determined by clotting factors, a type of enzyme which has the goal of converting a soluble protein in the blood known as fibrinogen into insoluble adhesive fibrin threads, which attach to the primary platelet plug and strengthen it into a clot known as a thrombus. Clotting factors are produced in the liver, and they are as follows:
Factor I (fibrinogen), Factor II (prothrombin), Factor III (tissue thromboplastin or tissue factor), Factor IV (ionized calcium), Factor V (labile factor or proaccelerin), Factor VII (stable factor or proconvertin), and Factor VIII (antihemophilic factor). Factors used in the coagulation cascade are Factor IX (plasma thromboplastin component), Factor X (Stuart-Prower factor), Factor XI (plasma thromboplastin antecedent), Factor XII (Hageman factor), and Factor XIII (fibrin-stabilizing factor).
The Extrinsic Pathway:
Tissue factor is found on the surface of many cells however usually outside of areas circulating blood. Therefore, when an injury is sustained, it comes into contact with the blood. Here it combines with factor VII and the complex formed activates factor X.
The Intrinsic Pathway:
This pathway involves different chemicals to the extrinsic pathway, however achieves the same means - activating factor X which triggers the final stage, the common pathway. It begins where subendothelial collagen (connective tissue) activates factor XII. This activates factor XI, then factor IX, which combines with factor VIII, calcium ions and platelet membrane phospholipids, and is then finally followed by factor X.
Stage Four: The Fibrin Plug Forms - (The Common Pathway):
After factor X has been triggered, it combines with factor V, calcium ions and platelet membrane phospholipids, which ‘wake’ the ‘dormant’ enzyme prothrombin; becoming thrombin, the crucial enzyme present here. This is what converts the fibrinogen into fibrin monomers, which polymerise into strands; ultimately strengthening the primary platelet plug into a thrombus. At the end of this stage, more thrombin is released, and this creates a positive feedback loop.
Blood clots and their production must be regulated however, as without the control of thrombin, clotting could spontaneously and violently occur, resulting in blockages of arterials - therefore heart attack and strokes. Therefore, many inhibitory proteins exist and circulate the body and are used to limit the production of thrombin once the coagulation cascade has served its purpose. Protein C is triggered very soon after the initial triggering of the release of thrombin, and it leads to the production of protein S. This protein begins to break down factors V and VIII - limiting the intrinsic pathway, therefore the production of thrombin.
After a wound has healed - new tissue has formed - fibrinolysis occurs. This is where an enzyme called plasmin breaks down the fibrin strands in the clot, which by this point would have shrunk and compressed, into smaller components, which can then be further broken down in fibrin degradation, before being naturally flushed from the body as waste.
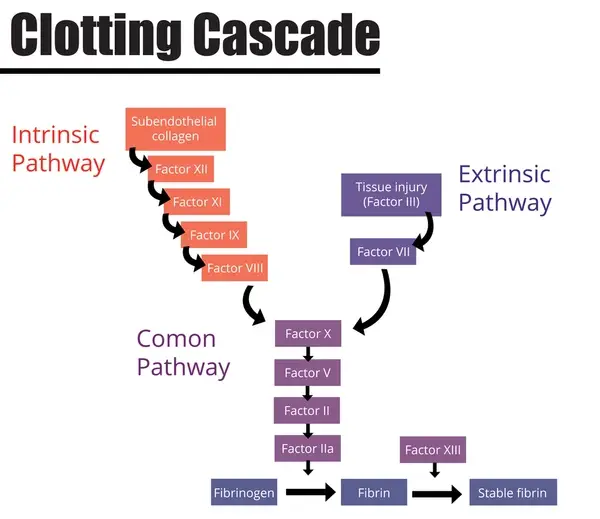
See above: Diagram displaying the events of the coagulation/clotting cascade.


Add comment
Comments